Things You'll Need
Jujubes(TM) candies
Round toothpicks
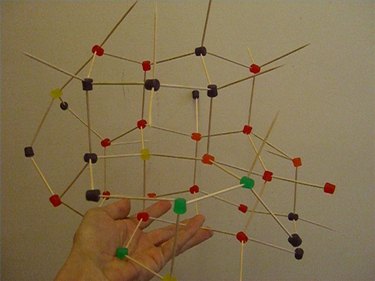
Diamond is made of nothing but carbon atoms. The geometrical arrangement of the atoms is what makes diamond so hard. You can make a model of the molecular structure of diamond using toothpicks and hard candies.
Step 1
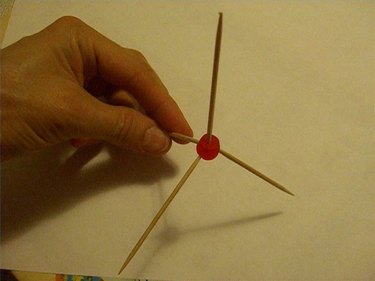
Use four toothpicks and one candy to make a tetragonal shape. The toothpicks should be coming out of the candy so that they are spaced exactly the same distance apart from each other. If you set this shape on the table, three of the toothpicks will form legs spread out in a triangle and the fourth toothpick will be pointing straight up.
Video of the Day
Step 2
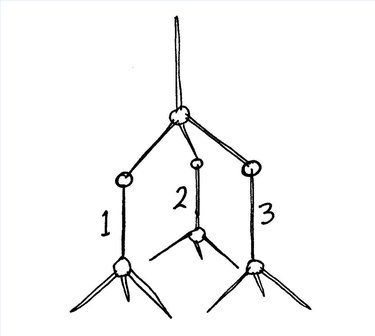
Make three more of these tetragonal shapes. Attach them to the first one so that their points are hanging down from the three lower toothpicks. You will need to add candies in the joints.
Step 3
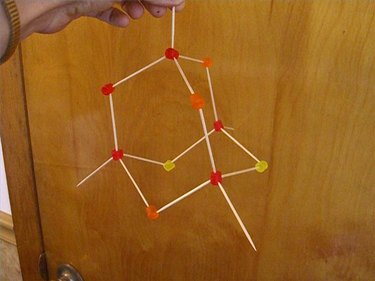
Continue this geometrical pattern where each candy has only four toothpicks coming out of it, with each candy connecting to four other candies. This simulates real science because each carbon atom bonds to four, and only four, other carbons.
Step 4
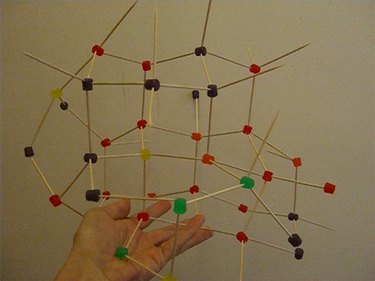
Keep adding more candies and more toothpicks, making sure that each candy is attached to exactly four others. (You will notice some hexagonal shapes emerging within the structure.) This pattern can go on forever, so when your model is as large as you want it, stop building.
Tip
Jujubes candies are superior to other hard candies for this project because of their consistency.
Video of the Day